How is a plasma protein therapy different?
Download as PDF
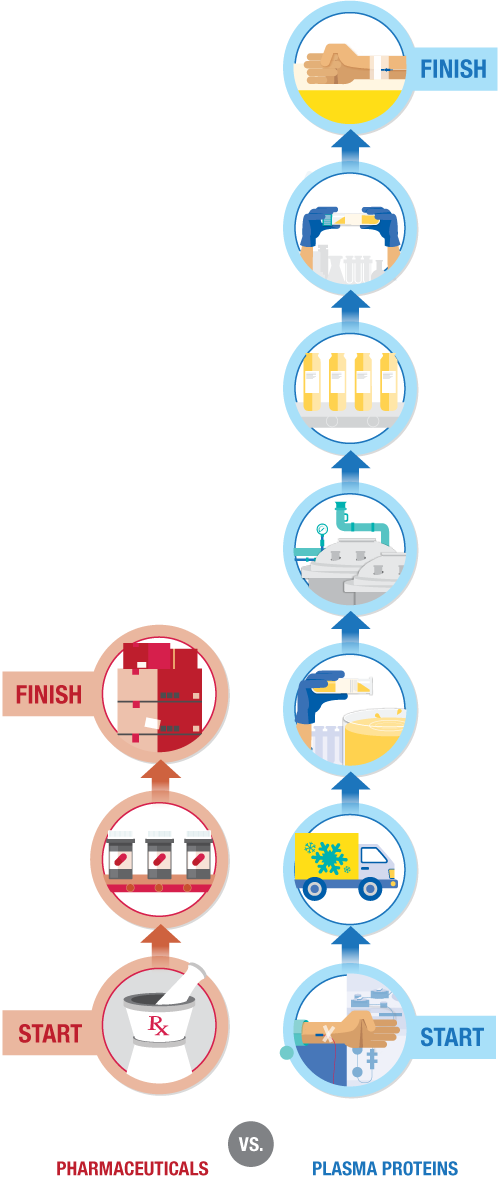
One-size-fits-all policies are unsuitable for plasma protein therapies. Although some value-based frameworks work for generic, interchangeable pharmaceuticals—a one-size-fits-all policy does not work for plasma protein therapies as these biologics are not interchangeable. Plasma protein therapies are high-impact pharmaceuticals because they increase life expectancy, improve quality of life, and reduce life-threatening complications for individuals with plasma protein deficiencies.
Human plasma or recombinant biological cell line
For most rare disease patients that rely on plasma protein therapies, the starting material is not an infinite resource. While some bleeding disorders can be treated using recombinant therapies, the majority of patients rely on therapies that are made using human plasma. Plasma cannot be made in a laboratory. Plasma and its lifesaving proteins can only be obtained from donors who so generously give their time to donate.- Pharmaceuticals Production starts with synthetic or chemical ingredients.
- Plasma proteins Production starts with a biological starting material, human plasma or recombinant biological cell line.